Your Cart is Empty

CuraCator Control the Application of Topical PRP
Trinnovations AESCURA
$195.00 - $1,080.00
Best Price Guarantee
At Medical Spa Supply, our mission is to empower Med Spa and medical wellness professionals with the equipment, supplies, and business solutions they need to thrive. One way we deliver on that promise is with our Best Price Guarantee. We want you and your team to shop with confidence, knowing you're getting the best possible price. If you find a lower delivered price on the same item from another authorized U.S. distributor for the medical wellness industry, chat, call, or email us before placing your order, and we’ll match it. We believe your time is best spent growing your business, not comparing prices.
xSupplies
Stock up and save Supply Orders over $250 Ship For Free!
xFlat Rate Ground shipping:
1st item: $8.50*
Additional items: $4.50 each
Once your cart reaches $250 in supplies Free Shipping
*$15 for bulky items or cases
Learn more about expedited shipping, special shipping cases, and international shipping at Shipping Policy
Equipment
Lowest Rates & Flat Rate Shipping Med Spa & Treatment Tables Ship for $199!
x- Small Equipment - $15 each item
- Medium Equipment - $35 each item
- Large Equipment - $100 each item
- XL Curbside - $199 each item
- XL Gold / Inside Delivery - $299 each item
- XL Platinum / White Glove - $599 each item
Learn more about these options expedited shipping, special shipping cases, and international shipping at Shipping Policy
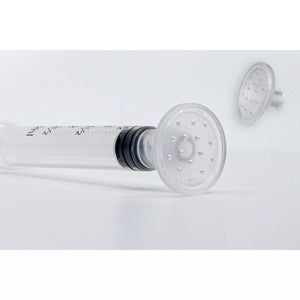
The CuraCator™ was designed and developed to meet the need for hands-free, needle-free, controlled application of products to skin or other tissue. It is a small disc-shaped device that affixes to a luer-lock syringe to help control the application of topical PRP. This alleviates safety concerns and risks of product waste or contamination. Dripping products such as platelet-rich plasma, exosomes, or other serums from syringes through a needle is dangerous. Applying products by dripping them onto tissue from an open-ended syringe leads to waste and little control. Wiping product onto tissue with a gloved hand can lead to contamination or adulteration of product. The goal of the CuraCator™ is to become the new standard of care for allowing product (whether liquid, cream, or ointment), to be safely and accurately applied to the skin or other tissue. Made from durable Triton plastic. FDA Class 1 Medical Device Accessory.
Comes in a Case of 20 Pieces
Before the CuraCator

Dripping expensive products such as platelet-rich plasma and exosomes onto the patient’s skin from an open-end syringe, or worse, from a needle. Without the CuraCator™ practitioners are at a huge risk for accidental needle sticks and, thus, exposure to infectious diseases
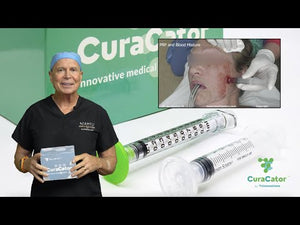
After the CuraCator

Using the CuraCator™ completely eliminates the risk of needle sticks and increases practitioner's safety. This FDA approved, patented design allows for precise and controlled application of topicals exactly where they're needed. This proven design means every drop of product is either used or contained and topicals are able to be confined to the allotted treatment area.
You may also like
Questions & Answers
Have a Question?
Be the first to ask a question about this.