Your Cart is Empty
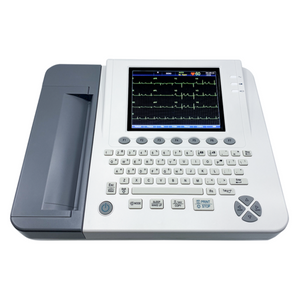










Edan SE-1200EB EKG/ECG Machine
Edan SE-1200EB
$2,495.00 - $3,664.45
Best Price Guarantee
At Medical Spa Supply, our mission is to empower Med Spa and medical wellness professionals with the equipment, supplies, and business solutions they need to thrive. One way we deliver on that promise is with our Best Price Guarantee. We want you and your team to shop with confidence, knowing you're getting the best possible price. If you find a lower delivered price on the same item from another authorized U.S. distributor for the medical wellness industry, chat, call, or email us before placing your order, and we’ll match it. We believe your time is best spent growing your business, not comparing prices.
xSupplies
Stock up and save Supply Orders over $250 Ship For Free!
xFlat Rate Ground shipping:
1st item: $8.50*
Additional items: $4.50 each
Once your cart reaches $250 in supplies Free Shipping
*$15 for bulky items or cases
Learn more about expedited shipping, special shipping cases, and international shipping at Shipping Policy
Equipment
Lowest Rates & Flat Rate Shipping Med Spa & Treatment Tables Ship for $199!
x- Small Equipment - $15 each item
- Medium Equipment - $35 each item
- Large Equipment - $100 each item
- XL Curbside - $199 each item
- XL Gold / Inside Delivery - $299 each item
- XL Platinum / White Glove - $599 each item
Learn more about these options expedited shipping, special shipping cases, and international shipping at Shipping Policy
The SE-1200 ECG Machine stands out for its exceptional cost-effectiveness,
seamlessly integrating precise clinical performance with a user-friendly interface.
With an on-board PDF creator facilitating effortless file transfers, this machine
ensures refined and dependable operation. Tailored to meet primary clinical
needs across diverse settings, it suits both adult and pediatric applications.
Features:
- 8” LCD screen
- USB support
- Alphanumeric keyboard
- Built-in thermal recorder
- Data transmitting to PC via LAN/WIFI
- Barcode scanner support
- PDF, SCP, FDA-XML, DICOM data export options
- One-touch operation with age and gender shortcuts
Edan SE-1200 EKG Machine
- Full Keyboard
- Glasgow Interpretation
- PDF Creator and USB Data Export
- Wi-Fi (Included)
- Stress Capable
- Touch Screen (Optional)
SE-1200EB Accessories:
- ECG Cable (4mm, banana connector) 01.57.471614
- Resting Tab Electrodes (PT-2334)
- 10-Lead Universal Clips (01.57.040172)
- Recording Paper (Z-fold, 210mm*295mm*) (SE-12P)
- Power Cord (USA standard) (01.13.036106)
- Rechargeable Lithium Battery (21.21.064149)
- User Manual
You may also like
Questions & Answers
Have a Question?
Be the first to ask a question about this.